First clinical trial of anticancer drug 'CAR-T' is underway for lymphoma patients
By ANI | Published: October 21, 2021 03:50 PM2021-10-21T15:50:23+5:302021-10-21T16:00:03+5:30
An anticancer treatment 'CAR-T (chimeric antigen receptor T-cell),' called a miracle anticancer drug, has been developed and the first clinical trial in Korea is being conducted.
First clinical trial of anticancer drug 'CAR-T' is underway for lymphoma patients
An anticancer treatment 'CAR-T (chimeric antigen receptor T-cell),' called a miracle anticancer drug, has been developed and the first clinical trial in Korea is being conducted.
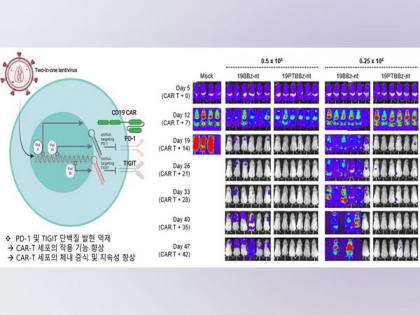
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 20th that a research team led by Professor Kim Chan-hyuk of the Department of Bioscience has transferred CAR-T Cell-based therapy technology to Curocell, co-founded by Professor Kim, and the clinical trial phase 1-b is being conducted on 10 patients with Diffuse Large B-cell Lymphoma (DLBL).
It is a stage of evaluating the safety and effectiveness of the treatment, and the clinical trial phase 2 will be conducted on 70 patients next year.
CAR-T cell therapy is a kind of immune anticancer drugs (drugs that use body's immune system to attack cancer cells) that genetically transformed to attack cancer cells by introducing CAR genes to T-cells, which are immune cells.
It is called a 'miracle anticancer drug' because it shows a high therapeutic effect of more than 80% in the clinical trials on patients with end-stage leukemia. (ANI/Global Economic)
( With inputs from ANI )
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor
Open in app