Union Health Secy to address States, UTs on COVID-19 vaccination of children aged 12-14 years tomorrow
By ANI | Updated: March 14, 2022 23:00 IST2022-03-14T22:53:57+5:302022-03-14T23:00:03+5:30
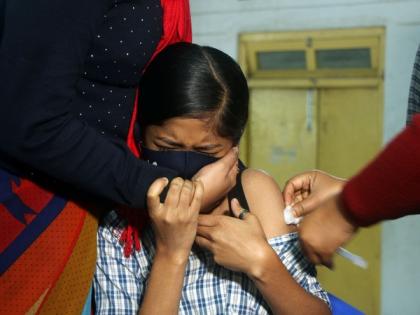
As COVID-19 vaccination of children aged 12-14 years are set to begin from March 16, sources on Monday informed that the Union Health Secretary Rajesh Bhushan will be addressing a Virtual Conference with States and Union Territories tomorrow (March 15).
Union Health Secy to address States, UTs on COVID-19 vaccination of children aged 12-14 years tomorrow
As COVID-19 vaccination of children aged 12-14 years are set to begin from March 16, sources on Monday informed that the Union Health Secretary Rajesh Bhushan will be addressing a Virtual Conference with States and Union Territories tomorrow (March 15).
As per the sources, the meeting would be on the vaccination of children aged 12-14 years and precaution dose for all citizens above 60 years of age at 11 AM.
Dr Sameer Bhati, Public Health Expert, Member Vaccination Awareness Committee, said, that India must celebrate this expansion of COVID-19 vaccination coverage making the kids of 12 to 14 years eligible for joining the safety shield against the pandemic.
"Corbevax has indeed joined the Covid vaccination campaign at the right time and shall surely become a relief for school-going children. We must involve the school authorities to expedite the vaccine coverage and reach out to every eligible child strengthening their circle of protection," he said.
"Moreover, it was extremely essential to protect our senior citizens amid the recent surge seen in China. Making all the above 60 population eligible for precautionary dose unconditionally will definitely maximize the protection against pandemic and increase the pace of our vaccination drive," Bhati added.
Further, Dr Naveen Parkash Gupta, a senior consultant of Neonatology at Madhukar Rainbow Children's Hospital said, "I don't have the data for those phase three trials, but as the government has approved, then the good efficacy has been found in those trials."
On the cost of the vaccine, he said, "it will be cost-effective there will be two doses which will be given intramuscularly."
Earlier today, Union Health Minister Dr Mansukh Mandaviya, in a tweet, said that COVID-19 vaccination of 12-14-year-olds and 'precaution dose' for all those above 60 years to begin from March 16.
Manaviya further said, "If children are safe then the country is safe!"
Notably, COVID-19 vaccination for the 15-18 year age group began on January 3.
As per the data of the Union Health Ministry, 3,37,70,605 children between 15 and 18 years of age group have received the second dose, while 5,58,92,605 have received their first dose of COVID vaccine.
Last week, Hyderabad-based pharmaceutical company Biological E has applied for Emergency Use Authorisation (EUA) for its COVID-19 vaccine Corbevax for the 5-12 year age group.
The data has been submitted by the company to the Subject Expert Committee for beneficiaries between 5-12 years of age.
Recently, the Subject Expert Committee recommended Emergency Use Authorization (EUA) for Biological E's COVID-19 vaccine Corbevax for the age group of 12 to 18 years under certain conditions.
( With inputs from ANI )
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor
Open in app